
Do you know how to regulate muscle stimulator at Anvisa?
The muscle stimulator is a health product highly sought after by athletes who want to complement their training and for people who do not like to go to the gym, the stimulator is an alternative to do workouts at home and tone your muscles.
To ensure the safety of these people, the muscle stimulator requires approval by Anvisa, the body responsible for regulating health products in Brazil.
Read more below and learn how to regulate the muscle stimulator at Anvisa.
What is muscle stimulator?
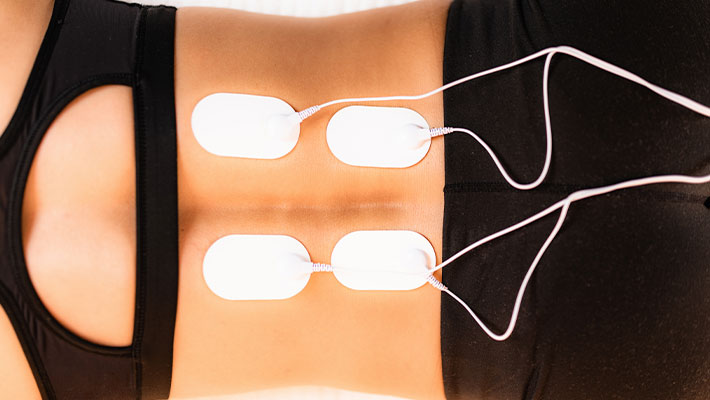
When we talk about physical exercise, we deal with an audience that likes and trains frequently, and with another type of audience that, to do daily practice, deals with many issues such as laziness, lack of interest and ends up not even doing it.
Thinking about this second example of public, the muscle stimulator was very successful outside of Brazil until it reached the Brazilian market. But it went further, not only people who do not exercise became interested in it, but also athletes, who use this type of product to complement their training.
The muscle stimulator is a muscle toning device, which aims to work on a specific region of the body through electrical stimulation that stimulates the muscle through electrodes fixed with the aid of a gel on the skin surface.
Through electrical pulses, the device burns fat. The muscle stimulator can be used at any time, for muscle toning; in addition it is considered as a practice of physical activity.
In this sense, the muscle stimulator is a device that must be regulated by Anvisa before reaching the market, aiming that it is not responsible for changing factors such as blood pressure and heart rate, ruling out any type of risk to the health of people who will use this product.
How is the regulation of the muscle stimulator done at Anvisa?
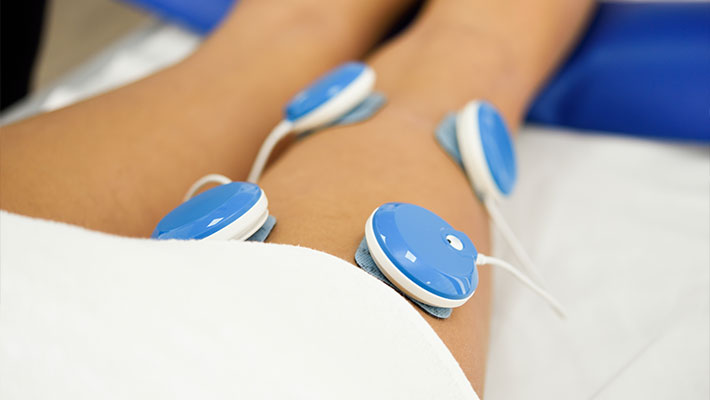
Anvisa is the regulator of health products, such as muscle stimulator. The manufacturer or importer of the product must first have his company registered as well as obtain the Operating License and AFE (Authorization for Operating Company – “Autorização de Funcionamento Empresa”), before regulating the muscle stimulator.
You can learn more about registering your company with Anvisa on our blog post: How to register your company at Anvisa.
In order to regulate the muscle stimulator at Anvisa, the manufacturer or importer needs to provide all product information. In this case, the product falls under risk class I, that is, it is a type of product considered to be of low risk. Therefore, according to Anvisa, it requires the approved notification to be commercialized.
The products classified as risk class I by Anvisa, are products that have characteristics that need proof of factors such as safety, care, restrictions, effectiveness, how to use, in addition to Inmetro certification that is carried out before the product is notified to Anvisa.
Likewise, the muscle stimulator is a type of product that requires simpler regulation due to its degree of risk. However, it is worth remembering that it is necessary for its regulation approved by Anvisa for any type of activity related to use or commercialization.
How to safely and quickly regulate the muscle stimulator?

Now that you know how to regulate the stimulator at Anvisa, you can count on our specialists to help you through the process. Currently, many companies choose to do the entire process alone and in the end end up bearing unnecessary losses of time and money.
Licempre has experienced professionals, with the aim of bringing less bureaucracy, greater time savings, money and headaches. The approval of a process at Anvisa makes your company offer more credibility and security for its consumers.
Do you want to know more about this and other regulatory issues? Access our blog or, if you prefer to receive contact from one of our specialists, contact us; soon we will return your question.


