
Do you know how to register a speculum at Anvisa? In this blog post, we will talk about the registration of health products, specifically about the speculum.
We remind you that all types of health products need to be certified or registered according to their risk class before Anvisa, before being sold.
Good reading!
What is the speculum?
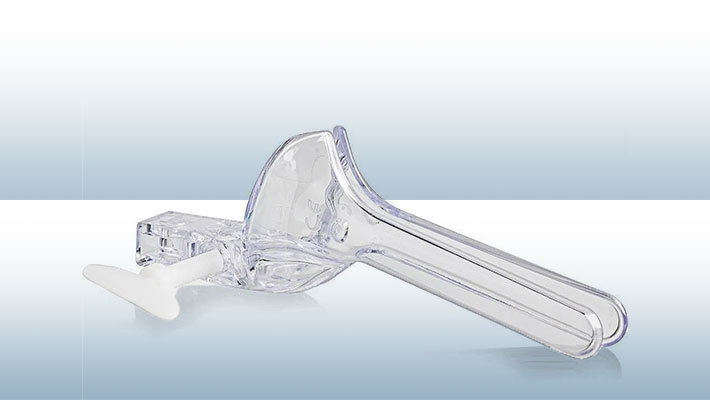
The speculum is a medical instrument used by health professionals for gynecological purposes where the doctor uses it to separate the vaginal walls. In this way the doctor can examine the inside of the patient’s cavity, in addition to collecting material for exams.
There are disposable specula that are most used, and reusable ones that in this case need to be sterilized for use.
Does the speculum need registration?

The speculum is a hospital material that, according to the Anvisa risk classification, belongs to risk class I or II.
In this way, it belongs to the group that has less risk and in this case there is no need to obtain the CBPF – Certificate of Good Manufacturing Practices. The manufacturer only needs to notify the product to Anvisa, following all regulations that the agency requires.
In summary, we can conclude that the product registration is for risk products III and IV, and for products of risk class I and II, notification is required according to RDC No. 423, OF SEPTEMBER 16, 2020.
Thus, the speculum needs notification at Anvisa in order to be marketed and then used by specialized health professionals.
What do I need to make the notification of the speculum in Anvisa?

Notification of the speculum at Anvisa is mandatory for the product to be marketed. Thus, it is important to remember that for this you need to be with your registered company, that is, you need the AFE of importer or manufacturer of the product, because only then you have access to the system for notification.
AFE is the Operating Authorization Certificate issued by Anvisa where it proves that the company is authorized to carry out the activities described in the certificate itself.
To learn more about company registration read our blog post: How to register your company at Anvisa.
How can Licempre help your company?

Licempre is a specialist in regulatory matters that aims to offer excellence in delivering results.
Our goal is to make the product or company registration process as less bureaucratic as possible with our experience and knowledge, bringing you more agility and security.
You can learn more about registering companies or other products by reading our blog content or if you have any questions speak directly to our experts.


